Penumbra (NYSE:PEN) has announced the completion of enrollment in the STORM-PE trial for its Lightning Flash system, a computer-assisted vacuum thrombectomy (CAVT) device. The trial, conducted in partnership with The PERT Consortium, aims to evaluate the effectiveness of CAVT plus anticoagulation against anticoagulation alone in treating acute intermediate-high risk pulmonary embolism (PE). The study successfully enrolled 100 patients for evaluation.
The STORM-PE trial seeks to provide high-quality evidence on the role of CAVT in improving right heart function and clinical outcomes in critically ill patients. Dr. Rachel Rosovsky, a hematologist at Massachusetts General Hospital, noted that the study aims to inform treatment guidelines and care for pulmonary embolism patients.

“This is an important milestone that underscores Penumbra’s commitment to transforming care for patients with pulmonary embolism,” said Dr. James F. Benenati, chief medical officer at Penumbra. “The trial successfully randomized patients well ahead of schedule thanks to the dedication of our clinical partners and the tireless efforts of our internal teams.”
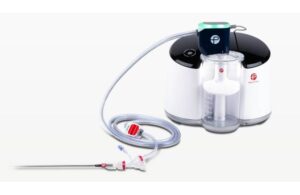
The Lightning Flash system is a mechanical thrombectomy device designed to address venous and pulmonary thrombus. It features Penumbra’s Lightning Intelligent Aspiration technology and advanced dual clot detection algorithms that utilize both pressure- and flow-based processes to detect blood clots and blood flow. The system’s catheter incorporates MaxID hypotube technology, allowing for a large inner diameter while maintaining a lower profile and soft, atraumatic tip design. This enables physicians to navigate complex anatomy and deliver high-power aspiration for effective clot removal.