Investments in global health research and development (R&D) have led to the development and launch of many life-saving health technologies over the past two decades. For instance, effective malaria vaccines didn’t exist twenty years ago, but today, two are being rolled out across Africa. The future of global health R&D looks promising with a robust pipeline of candidate products, although some neglected diseases such as leprosy, scabies, and trachoma still lack sufficient candidates.
In theory, the next twenty years could be even better than the last for launching breakthrough global health technologies. However, several headwinds are currently impeding the swift delivery of new products to the market. Development timelines can be lengthy, success rates are low, and the cost of late-stage clinical trials is soaring.
Could innovations in the R&D ecosystem help overcome these hurdles and drive more efficient product development? Two new studies from our research team aim to answer that question. Our research involved over 120 expert interviews, a review of existing literature, and the application of quantitative modeling tools to estimate how innovations in the R&D ecosystem could affect the costs, success rates, and overall impact of future R&D efforts.
Six Shifts in the R&D Ecosystem Could Drive Efficiencies
The first study identified six ecosystem innovations that could potentially make global health R&D faster and more efficient. These include:
-
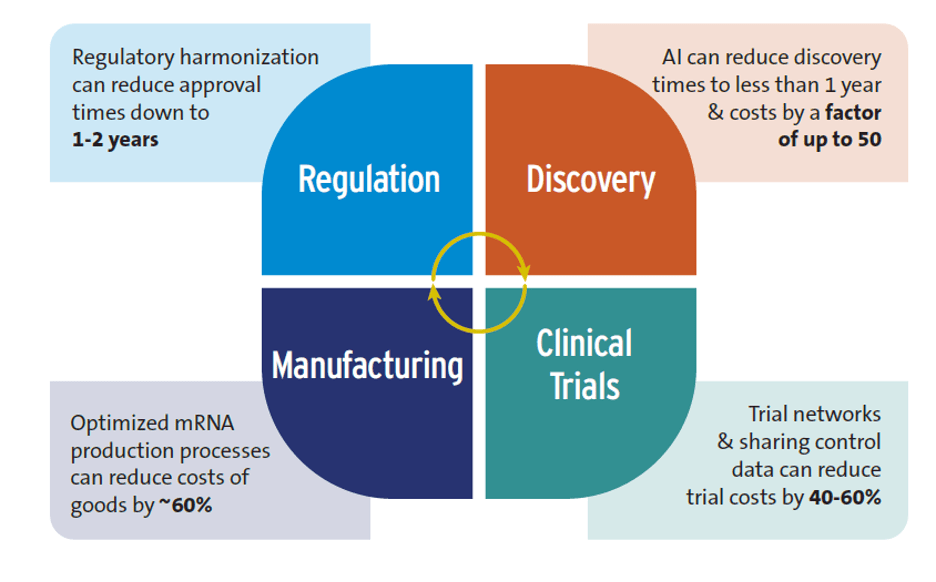
Artificial Intelligence (AI): While some claims about AI are exaggerated, such as those about replacing human clinical trials, evidence indicates that AI is already having a positive impact on global health R&D, particularly in the early stages of the research process. According to studies, the R&D discovery phase can be shortened to less than 12 months, and discovery costs lowered by a factor of 50. It’s essential to consider, however, that the inequitable rollout of AI could worsen inequalities between high-income and low- and middle-income countries.
-
Advances in trial design: Clinical trial networks, critical in developing both COVID-19 and mpox vaccines, are a good example here. These networks save time and money by using existing sites, recruiting patients more quickly, along with sharing control groups which can reduce costs by 40-60%, according to studies.
-
New techniques for manufacturing drugs and vaccines: Using improved mRNA production approaches can lower costs. Optimized mRNA production processes could save over 60% of the annual cost of goods for producing 100 million vaccine doses compared to conventional mRNA manufacturing. Such savings could decrease mRNA vaccine production costs to just $0.5 per dose.
-
Accelerating regulatory approval of new health technologies: One factor adding to the long delay in health product market authorization involves the substantial time gap between HICs and LICs, with the lag time between the initial submission for regulatory approval and final approval in sub-Saharan Africa estimated at 4-7 years. Regulatory harmonization can reduce approval times to 1-2 years.
-
New types of health technologies, such as mRNA and monoclonal antibodies: The versatility of mRNA platforms has been shown during the COVID-19 pandemic, highlighting their speed, which is especially valuable during pandemics. However, the patent holders for many production inputs are in HICs; overcoming this barrier will require stronger sharing of intellectual property and technology transfer agreements.
-
Financing innovations: These innovations are being used to address the constrained funding environment for global health R&D. An example is the U.S. priority review voucher (PRV) scheme, which incentivizes developers of new health products for eligible neglected diseases. The scheme has supported the development of new health tools for global health conditions and could be beneficial for the European Union as well.
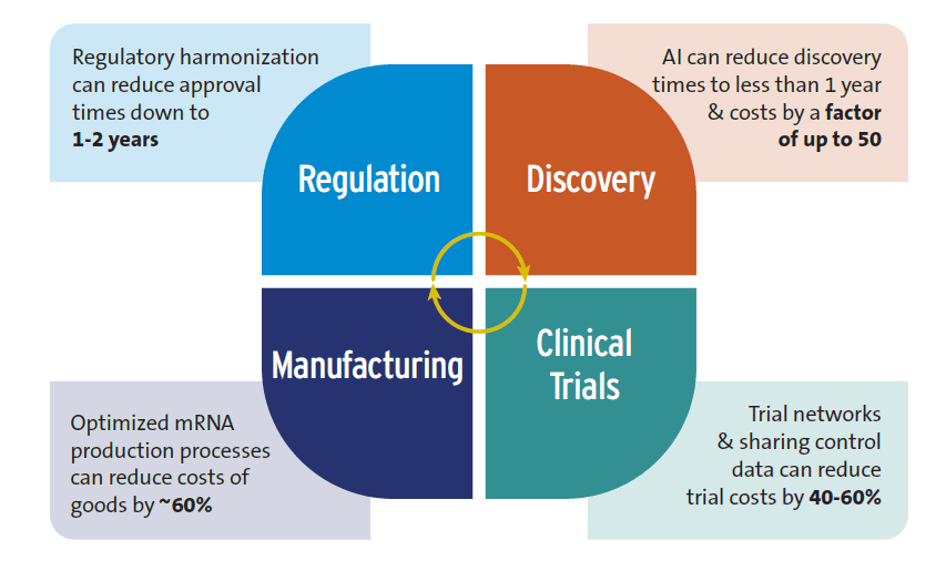
Figure 1. Potential efficiency gains from shifts in the R&D ecosystem Source: Authors’ research
Modeling the Impacts of These Efficiencies
The second study used quantitative models to estimate the impact over a 22-year period (2023-2044) if some of the key innovations were widely adopted. One metric used was societal net monetary benefits (NMBs), calculated as the difference between the economic value of the health benefits and the incremental costs associated with the product development. If ecosystem innovations are not adopted, current investments would yield an estimated 453 product launches, with only 42 of those yielding positive NMBs.
Adopting ecosystem innovations could lead to the launch of all 94 needed products, and the number of products with positive net monetary benefits would increase. The incremental cost, over and above current spending, would be an additional $1.4 billion to $7 billion annually. Combining pooled investments with AI and smarter clinical trial designs would result in savings of over $9 billion, and a reduction in the average cost per product launch by up to $100 million. The number of products yielding positive NMBs would increase even further if market entry was faster and production costs were lower, leading to an increase to 115 products with positive NMBs.
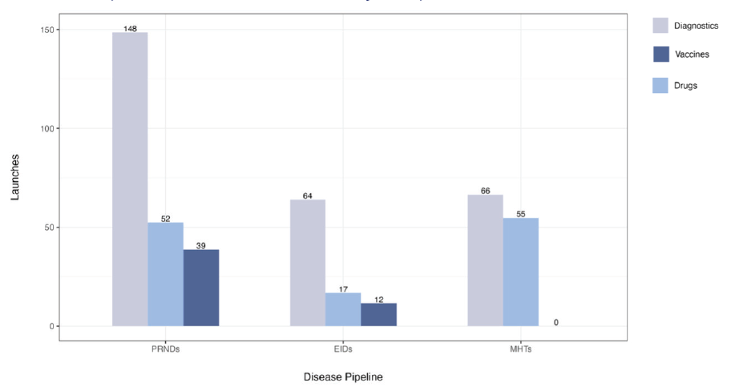
Figure 2. Potential product launches from the current product development pipeline Note: PRNDs: poverty-related and neglected diseases; EIDs: emerging infectious diseases; MHTs: maternal health technologies. Figure assumes no replenishment and no adoption of ecosystem innovations. Source: Authors’ research
In conclusion, the widespread adoption of innovations in the R&D ecosystem, encompassing everything from AI to smarter product development approaches, could make critical health tools more accessible and affordable. Our two new reports suggest we could be on the cusp of a revolution in global health R&D.
Gavin Yamey, Director, Center for Policy Impact in Global Health and Professor of Global Health – Duke Global Health Institute, Duke University

Shingai Machingaidze, Co-chair, 100 Days Mission Science and Technology Expert Group – International Pandemic Preparedness Secretariat

Osondu Ogbuoji, Deputy Director, Head of Research, Center for Policy Impact in Global Health – Duke Global Health Institute, Duke University

Marco Schäferhoff, Managing Director – Open Consultants