Revolutionizing Global Health R&D: How Innovations Can Accelerate Life-Saving Technologies
Investments in global health research and development (R&D) have yielded remarkable results, leading to the creation of life-saving technologies. Two decades ago, a malaria vaccine was nonexistent; now, effective vaccines are being distributed across Africa. The future appears promising, with a robust pipeline of potential products for many global health conditions. However, some neglected diseases, such as leprosy, scabies, and trachoma, still have limited candidate products.
In theory, the next twenty years could surpass the achievements of the previous two in terms of breakthroughs in global health technologies. Yet, significant challenges hinder the rapid market introduction of new products. The timelines for discovery, clinical trials, and regulatory approval are frequently extended, particularly in low- and middle-income countries (LICs and MICs). Success rates remain low; a study indicated that only around 1 in 20 drug development projects results in a product launch. Furthermore, late-stage clinical trials are increasingly expensive, with a Phase 3 trial of a tuberculosis vaccine candidate costing over $500 million. Meanwhile, the annual funding for R&D related to neglected diseases is declining.
Could innovations in the R&D ecosystem—new methods of discovering, testing, manufacturing, and financing health technologies—help overcome these obstacles and foster more efficient product development? Two recent studies address this question. Conducted through over 120 expert interviews, literature reviews, and the use of quantitative modeling tools to estimate the impact innovations in the R&D ecosystem could have, the studies evaluated their effects on the costs, success rates, and overall impact of future R&D efforts.
Six Shifts in the R&D Ecosystem
The first study revealed six ecosystem innovations with the potential to accelerate and enhance the efficiency of global health R&D.
Artificial Intelligence (AI)
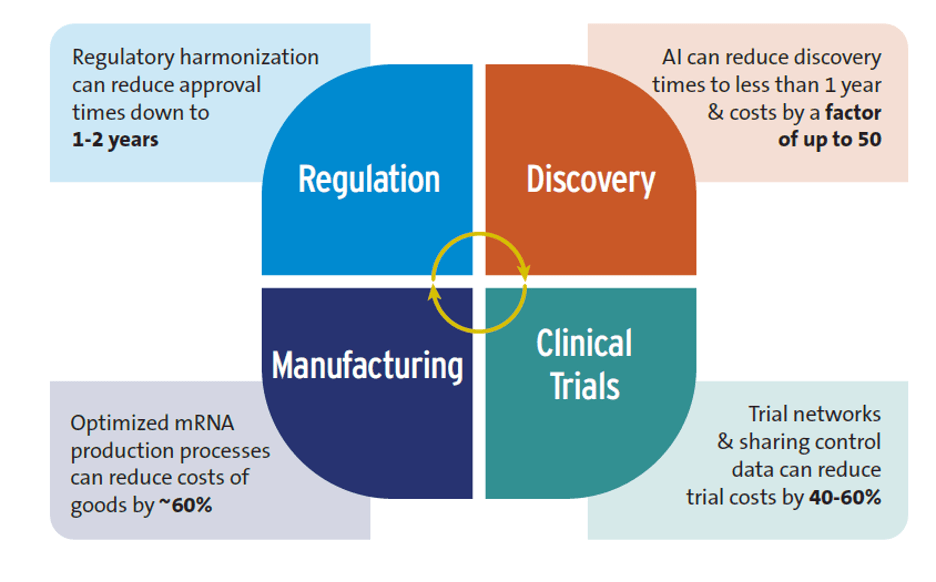
Despite some overoptimistic claims—for example, that AI will replace human clinical trials—the research showed that AI is already positively impacting global health R&D, particularly in the early stages. The discovery phase of R&D typically requires 3-5 years and can cost up to $10 million, but AI can reduce this to less than 12 months and lower costs by a factor of 50. However, interviewed experts cautioned that AI could exacerbate inequalities between high-income countries (HICs) and LICs and MICs if not implemented equitably.
Advances in Trial Design
Clinical trial networks, instrumental in developing COVID-19 and mpox vaccines, offer a good example. These networks save time and money by utilizing existing trial sites rather than creating new ones. They also facilitate quicker patient recruitment and reduce the patient pool by sharing control groups. Costs could be reduced by 40-60% by sharing control groups and using control data from previous trials.
New Techniques for Manufacturing Drugs and Vaccines
Modular manufacturing facilities have a smaller footprint than traditional plants, which leads to lower capital costs. Improved mRNA production methods can also reduce expenses. Optimizing mRNA vaccine production could save more than 60% annually on the cost of goods for 100 million vaccine doses compared to conventional mRNA manufacturing. Such savings may decrease mRNA vaccine production costs to as low as $0.5 per dose.
Accelerating Regulatory Approval of New Health Technologies
There is a significant delay in market authorization between HICs versus LICs for new health products. One study found a 4-7 year lag between first submission of new medicines for regulatory approval (usually to a regulator in a HIC) and final approval in sub-Saharan Africa. Regulatory reform consisting of regulatory harmonization (e.g., countries performing joint regulatory reviews), strengthening national and regional regulatory capacity, and conducting rapid scientific reviews are all contributing to shortening this gap. Regulatory harmonization can reduce approval times to 1-2 years.
New Types of Health Technologies
The COVID-19 pandemic highlighted the versatility and speed of mRNA platforms, which is particularly valuable during pandemics. Ensuring that LICs and MICs can manufacture their own mRNA technologies will be critical. However, since the intellectual property holders for many mRNA production inputs are primarily in HICs, overcoming this barrier will necessitate enhanced agreements for sharing intellectual property and technology transfer.
Financing Innovations
New financing approaches are being used to address the challenging funding environment for global health R&D. For example, the U.S. priority review voucher (PRV) scheme incentivizes developers of new health products for neglected diseases with a tradeable voucher, which grants them priority review for a second product candidate, with the voucher worth around $100 million. This scheme has supported the development of new health tools for global health conditions, and implementing a similar PRV scheme in the European Union may prove beneficial.
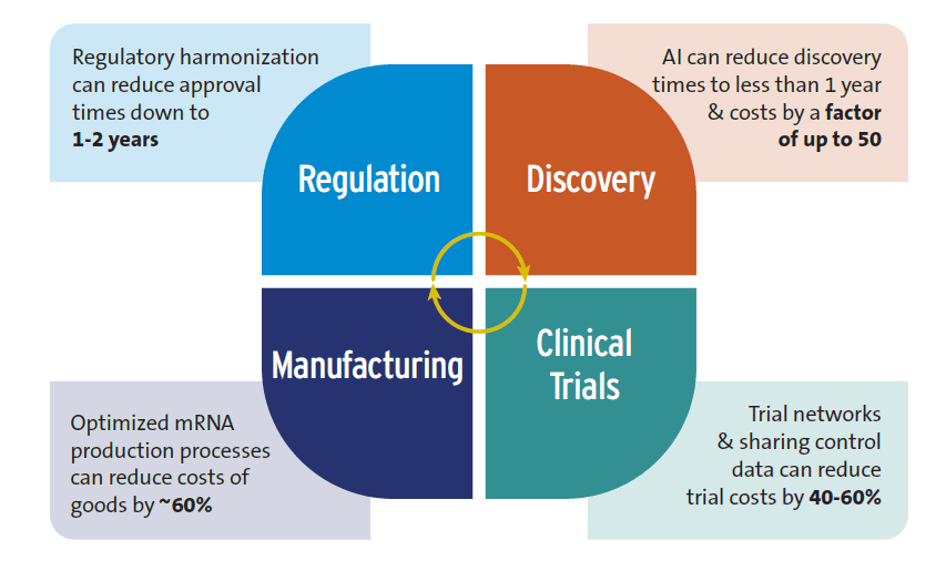
Figure 1 summarizes the potential of these innovations to accelerate R&D and lower its costs.
Modeling the Impacts of These Efficiencies
The second study utilized quantitative models to estimate outcomes over a 22-year period, spanning from 2023 to 2044, if key R&D ecosystem innovations were widely adopted. Societal net monetary benefits (NMBs) were one metric used. Calculated as the difference between the economic value of health benefits derived from a successful product launch and the incremental costs linked with developing, launching, and delivering the product to the target population, it provided insight into potential gains.
Currently, there are 1,498 candidate medicines, vaccines, and diagnostics in the product development pipeline for neglected diseases, emerging infectious diseases, and maternal health conditions. Without adopting ecosystem innovations and assuming no replenishment of the current pipeline, investing in R&D to advance the current candidates would yield an estimated 453 product launches between 2023 and 2044. Of these products, 42 would yield positive NMBs.
However, 94 urgently needed products would remain unlaunched, for example, new treatments and diagnostics for maternal iron deficiency anemia and fetal distress, and vaccines against six emerging infectious diseases (EIDs), including Crimean-Congo Hemorrhagic fever, Ebola, Marburg, Nipah, Rift Valley fever, and Zika.
How would this picture change if ecosystem innovations were adopted? Pooling and coordinating investments could lead to the launches of all 94 needed products, and the number of products yielding positive net monetary benefits would increase from 42 to 106. The incremental annual cost, beyond current R&D spending, would range from $1.4 billion to $7 billion, depending on the complexity of the product candidates. Combining pooled and coordinated investments with AI and smarter clinical trial designs could result in savings exceeding $9 billion and a reduction in the average cost per product launch by up to $100 million. The number of products yielding positive NMBs would increase to 110. Even greater gains would be achieved if market entry was accelerated and production costs were lower; the number of products yielding positive NMBs would increase to 115.
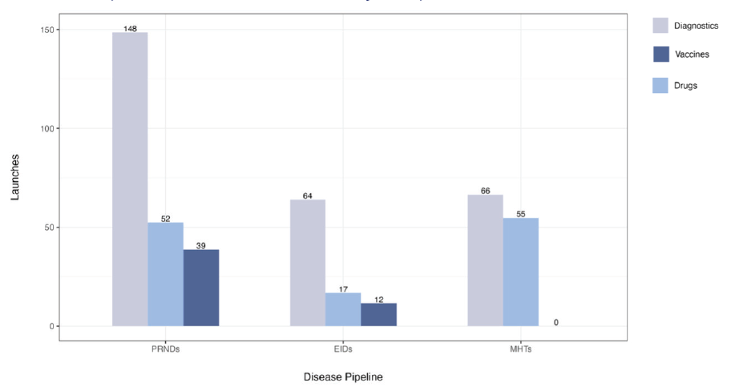
Figure 2. Potential product launches from the current product development pipeline
Note: PRNDs: poverty-related and neglected diseases; EIDs: emerging infectious diseases; MHTs: maternal health technologies. Figure assumes no replenishment and no adoption of ecosystem innovations.
In conclusion, broadly adopting a range of innovations, from AI to smarter product development approaches, could expedite the availability of critical health tools and reduce associated costs. The two new reports suggest that a revolution in global health R&D is potentially underway.
Authors: