The Rise of Sensor-Based Digital Health Technologies
Sensor-based digital health technologies (sDHTs) are revolutionizing how we collect clinical data. These technologies offer the unique ability to gather high-resolution information in real-world, remote settings over extended periods, providing insights into meaningful functional changes with reduced observer bias. Between 2019 and 2024, there has been a tenfold increase in the adoption of sDHT-derived measures in industry-sponsored interventional trials, with over 100 trials using these measures as primary endpoints. Recent milestones include the FDA’s endorsement of a digital measure as a primary endpoint in a pivotal trial in 2023, and the EMA’s qualification of a digital measure for primary efficacy. Furthermore, the FDA has qualified atrial fibrillation burden as the first medical device development tool captured by an sDHT.
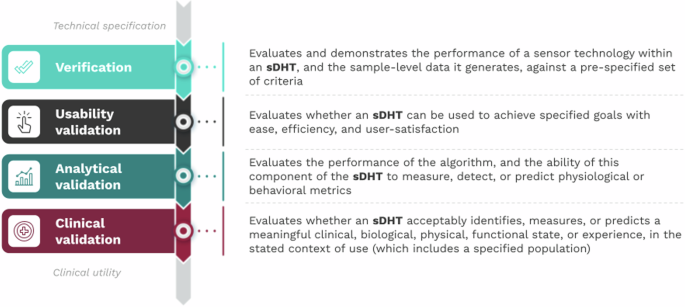
While the integration of sDHTs for remote patient monitoring and clinical practice has been a gradual process, it has accelerated due to the development of more robust infrastructure designed to handle the complexities of large healthcare systems. This increasing trust and investment from healthcare providers, study sponsors, regulators, payers, and patients has been supported by the V3 framework. V3 assesses the quality of sDHTs through three components: Verification, Analytical validation, and Clinical validation.
Since its publication in 2020, the V3 framework has become a cornerstone for evaluating sDHTs’ technical, scientific, and clinical performance and has been adopted and referenced widely by leading regulatory and research groups.
Extending V3: The Need for Usability Validation
In the four years since the V3 framework was launched, regulations concerning sDHTs for remote data collection have advanced, along with the development of corresponding reimbursement pathways. Moreover, the widespread use of general wellness products has empowered individuals to monitor their own health, shaping the expectations for sDHT implementation in research and healthcare settings.
As clinical research sponsors and healthcare organizations implement digital clinical measures at scale, challenges related to integrating sDHTs across different populations, settings, and methodological approaches have become more apparent. Real-world examples, such as the loss of tremor classification data in a Parkinson’s Disease study due to device permission issues, and the FDA recall of a blood glucose monitor due to unit switching errors, highlight the need for a unified set of best practices to ensure optimal sDHT usability. Usability is defined as the extent to which an sDHT can be used to achieve specified goals with ease, efficiency, and user satisfaction.
To account for the heterogeneity of sDHTs and to support the development of appropriate solutions, a new framework, called V3+, builds upon V3 by incorporating an evidence-based component that addresses usability validation. This extension ensures sDHTs can be developed and evaluated using modern human factors engineering approaches for the creation of high-quality, well-validated, and user-friendly digital measures to inform scientific, clinical, regulatory, and payer decision-making.
The V3+ Framework: A Focus on Usability
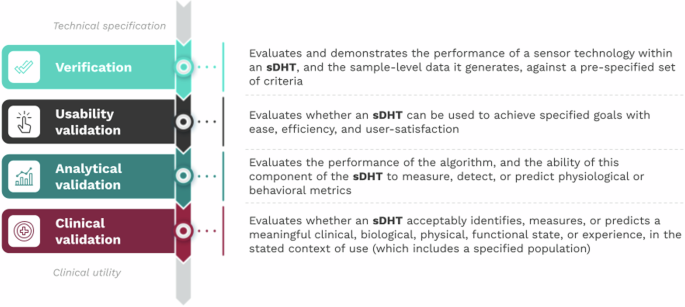
The V3+ framework includes four components, with usability validation as the most recent addition. Though sequential, V3+ is a modular structure.
The V3+ framework applies to sensor-based digital health technologies (sDHTs), defined as connected digital medicine products that process data captured by mobile sensors using algorithms to generate measures of behavioral and/or physiological function. sDHTs can include systems that use computing platforms, connectivity, software, and/or sensors, for health care and related uses.
Four Key Activities
The usability validation component of V3+ is composed of four key activities, which begin with a proposed intended use statement that defines the clinical circumstances or purpose for which the sDHT is being developed.
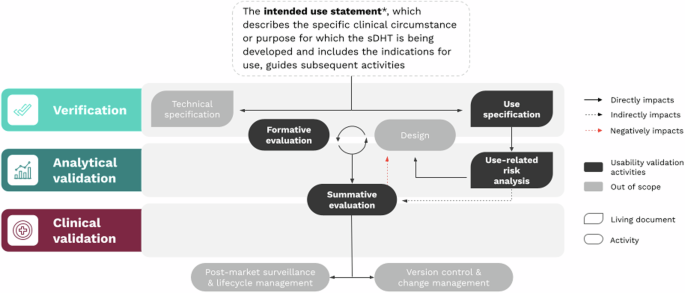
1. Develop the Use Specification
The intended use statement informs both the technical specification (detailed description of the sDHT) and the use specification (who the users are, how they interact with the sDHT, and their motivations). The use specification is the counterpart to the technical specification, of equal importance and with a bidirectional relationship.
2. Conduct a Use-Related Risk Analysis
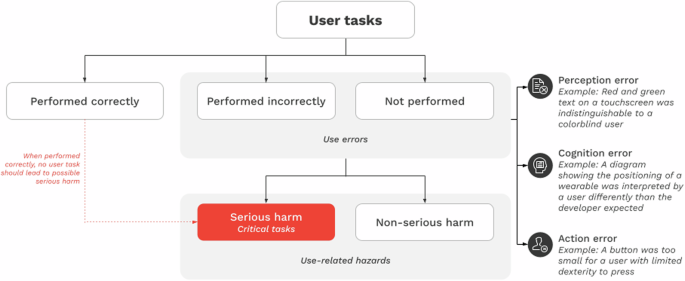
The risk analysis identifies foreseeable risks associated with the sDHT’s use and develops steps to minimize those risks, including misuse and use-errors. The analysis should consider all potential use-errors and hazards.
3. Conduct Iterative Formative Evaluation of sDHT Prototypes
Formative evaluations involve activities to describe user tasks, identify use-errors, and gather information to improve design. The evaluation process is iterative, with revisions that incorporate user feedback. The aim is to refine prototypes.
4. Complete Summative Evaluation of the Final sDHT
Summative evaluations (or human factors validation studies) verify that the final version of the sDHT is usable within the proposed intended use. These studies should evaluate all essential user tasks, components of the user interface, and user groups. Quantitative pass/fail criteria should be specified beforehand.
Pre- and Post-Market Applications of Usability Validation
Similar to the original V3 framework, the V3+ usability validation is applicable to two use cases: pre-market and post-market technologies.
For pre-market (under development) sDHTs, the technology developer handles the processes described in V3+. Pre-market processes of regulated medical devices follow the regulations and guidelines, which is not the case for sDHTs that are not marketed as medical devices.
For post-market (commercially available) technologies, the party who deploys the sDHT—either the clinical research study sponsor or the healthcare provider—is responsible for ensuring the product has been satisfactorily assessed by V3+. Stakeholders are advised to work with the sDHT developer to enhance future products and ensure that previously-unidentified use-errors are fixed.
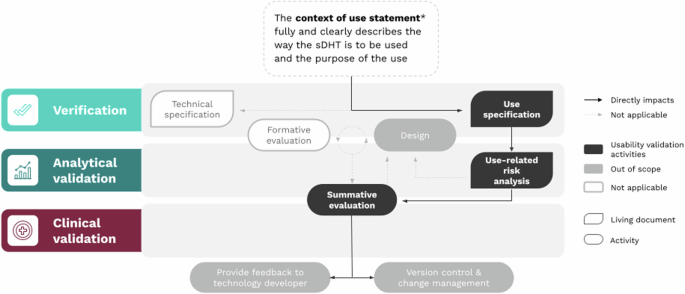
The focus of usability validation in a post-market sDHT environment considers a gap analysis that compares the original intended use with the current context of use. The aim is to identify if the current intended context aligns with the original development, and the results inform further evaluation procedures.
Integration and Future Directions
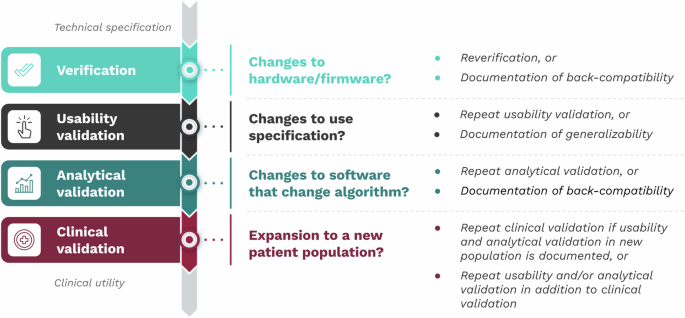
As the V3+ framework is modular, changes to one component don’t necessarily require new data collection in the other components. This flexibility enables efficient evidence generation. The original components of V3—verification, analytical validation, and clinical validation—remain unchanged.
The rapid expansion of sDHTs for clinical research and digital health necessitates a consistent vocabulary and a standardized method for usability validation that meets global regulatory guidelines and industry standards. V3+ highlights the need to take a multidisciplinary method to usability validation that incorporates user experience. By developing recommendations for usability validation best practices and supporting their implementation, sDHT user experience can become a key product differentiator, which will ensure optimal care for diverse patient populations.