Insights from the European Medicines Agency on Digital Health Technology Derived Endpoints
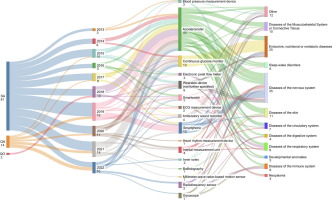
The European Medicines Agency (EMA) has been evaluating the use of digital health technologies (DHTs) for endpoint measurement in clinical trials. A recent study analyzed Qualification Opinions, Qualification Advice, and Scientific Advice procedures issued by the EMA between 2013 and 2022 to understand how DHTs are being utilized.
Key Findings
- Most Common DHTs: Accelerometers are the most frequently proposed DHTs, followed by glucose monitors and smartphones.
- Application Areas: DHTs are often used in trials for nervous system diseases, particularly for mobility measures and objective testing.
- Primary Use: Most DHTs are proposed for efficacy endpoints, indicating their potential in measuring treatment effectiveness.
- EMA Feedback: The EMA emphasizes the importance of validation, precision, and a clearly defined context of use for DHTs in clinical trials.
EMA’s Action Plan
The EMA has outlined an action plan to support the advancement of DHT methodologies in clinical trials. This includes providing training and updating guidance on novel methodologies.
Conclusion
The integration of DHTs in clinical trials represents a significant advancement in drug development. By providing novel endpoint measurements, DHTs can enhance the efficacy and objectivity of clinical trials. The EMA’s proactive approach in guiding the use of DHTs is crucial for their successful integration into drug development processes.