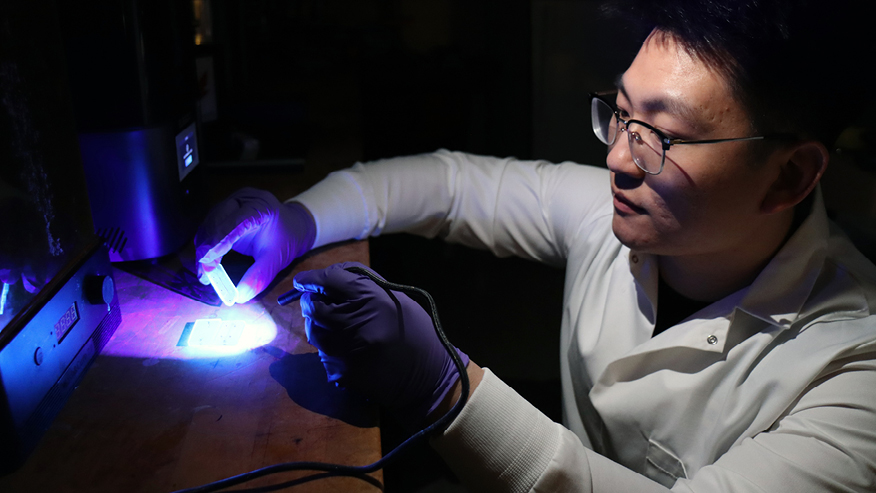
Purdue-Affiliated Startup Launches Cutting-Edge Crystallization Solutions
WEST LAFAYETTE, Ind. — A new high-tech startup, Crystallization Systems Technology Inc. (CrySyst), has emerged to revolutionize processes within the pharmaceutical and fine chemical industries. The company, founded by international process systems and operation experts, aims to streamline and enhance manufacturing through its innovative quality-by-control (QbC) framework.
CrySyst’s software solutions focus on crystallization monitoring, modeling, and control. Their tools are built upon extensive research published in prominent journals, including Crystal Growth & Design and Industrial & Engineering Chemistry Research. A major figure behind CrySyst is Zoltán Nagy, the Arvind Varma Professor of Chemical Engineering at Purdue University’s Davidson School of Chemical Engineering, along with co-founder Botond Szilágyi, formerly a postdoctoral research associate at Purdue and now an associate professor at Budapest University of Technology and Economics.
CrySyst has been granted a license by the Purdue Innovates Office of Technology Commercialization to commercialize its proprietary technologies, CryMoCo and CrySiV. These software solutions represent a significant advancement in the field.
Addressing Key Challenges in Process Development
According to Nagy, the primary hurdles in model-based process development involve identifying necessary experiments, selecting appropriate model structures, and obtaining reliable model parameters. “CrySyst’s tools directly address these pain points by providing guided experiment selection, offering a semiautomated framework for model development, and delivering reliable, high-confidence solutions,” Nagy explained.
CrySiV and CryMoCo offer a systematic and scientifically rigorous approach to crystallization process development. Nagy emphasized that, “These tools reduce time, material usage and risk while enhancing process robustness and scalability.” This QbC approach facilitates the rapid design of robust crystallization processes.
CryMoCo is vendor-independent crystallization process monitoring and control software. It integrates industry-standard communication protocols with process analytical technology and state-of-the-art control methods, including direct nucleation and supersaturation control. CrySiV is a user-friendly crystallization simulator that employs population balance modeling for the digital design of processes. It includes kinetic parameter regression and visualization, and features process optimization capabilities for both crystallization and integrated crystallization-wet milling processes, utilizing advanced numerical methods.
Nagy highlighted the benefits of CryMoCo and CrySiV, including:
- An intuitive and user-friendly interface, decreasing the learning curve for industry professionals.
- State-of-the-art numerical solvers, providing reliable and reproducible solutions, including rigorous model validation.
- A structured workflow, guiding users through model selection and refinement, while minimizing computational uncertainties.
Industry Impact and Future Outlook
Pharmaceutical and fine chemical companies often face process development limitations due to restricted material availability, constraints on experimental resources, and lengthy optimization timelines. Nagy noted that “These challenges can be effectively addressed through model-based digital design and model-free direct design approaches, which have seen increasing adoption.” However, systematic workflows and robust tools are crucial for widespread industrial implementation, which CrySyst aims to provide.
“We are committed to helping our clients develop and scale their process technologies faster, using less material. We offer specialized software products for model-free and model-based crystallization process design as well as consultancy and training services for problem-solving with our software tools,” Nagy stated.
CrySyst’s development was supported by funding from the Enabling Technologies Consortium, which included over ten major pharmaceutical companies. “We collaborated on a project with them for three years and, through close interaction with the crystallization scientists from the member companies, we developed the tools based on feedback we received to fit the needs of the industry,” Nagy said.