The Expanding Role of Digital Health Technologies in Modern Healthcare
Digital health technologies (DHTs) – encompassing mobile health (mHealth) solutions, health information technology, telehealth and telemedicine, and personalized medicine – are becoming increasingly prevalent in medical practice globally. However, the adoption, regulatory approval, and reimbursement of these technologies vary significantly across different countries. Notably, DHTs are poised to play a critical role in the economic affordability and environmental sustainability of healthcare systems, addressing previously unmet societal needs. These technologies offer customized, adaptable care solutions, facilitating safer and more extensive home-based and local care, thereby empowering patients to actively manage their own health.
This article, the first in a two-part series, explores the evolving landscape of flexible DHT suites, current regulatory and Health Technology Assessment (HTA) frameworks, and their practical applications, particularly during the COVID-19 pandemic. The subsequent article in this series will delve into potential future frameworks.
The Emergence of DHT Suites
The COVID-19 pandemic accelerated the bundling of individual DHTs, each with specific intended medical uses and associated approvals, into suites designed to deliver aggregated ‘intended purposes.’ These purposes sometimes differ significantly from those of individual component devices within the suite. This complexity, characterized by intricate interactions both within and between device suites, presents novel opportunities for medical treatment while also creating the potential for unexpected “emergent” properties. DHT suites can integrate with telemedicine and electronic health records (EHRs) at varying levels. Examples of services delivered via aggregated DHT devices include remote sensor systems, automated monitoring platforms, alarms, and teleconsultation platforms to:
- Allow healthcare providers (HCPs) to monitor chronically ill patients at home.
- Enable Hospital-at-Home programs (HaH).
- Improve treatment efficiency in hospital or care home settings.
- Enable ‘smart clinics’ with remote examinations.
This exploration examines the challenges associated with implementing, evaluating, and assessing the interactions within grouped, networked DHT systems for both regulatory and HTA purposes.
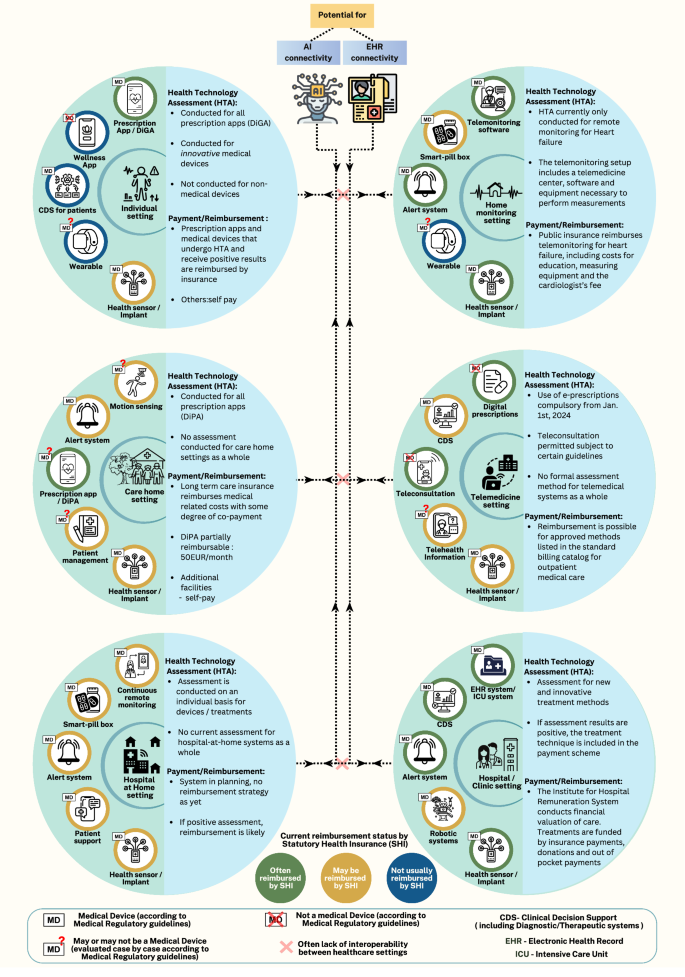
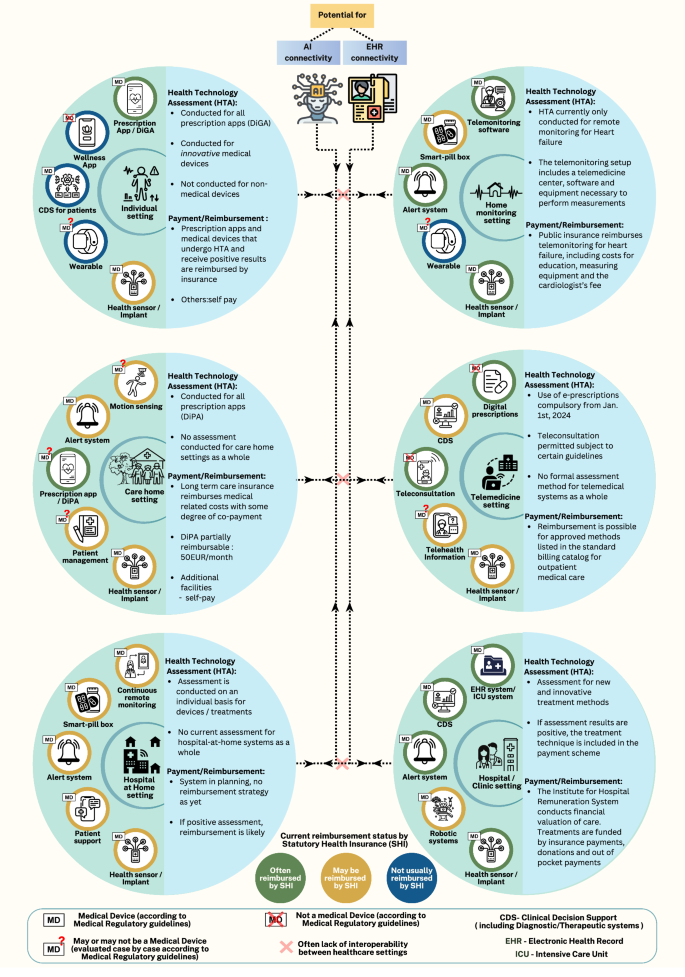
From Isolated Devices to Integrated Systems
The practice of grouping medical devices, whether from the same or different manufacturers, is not new. However, the use of DHT aggregations is evolving from simple groupings toward fully networked and interconnected devices. These systems are characterized by complex data flows and dynamic dependencies. While many suites still depend on healthcare staff for operation, automation trends suggest a future of ‘super devices’ with limited human intervention.
Hospital-at-Home: A Case Study in DHT Evolution
The Hospital-at-Home (HaH) concept, as shown in the included figure, typically comprises aggregated health sensors, alert systems, smart pill boxes, systems that facilitate remote monitoring, and provides digital patient support. HaH suites, already used for treating patients, have established regulatory pathways. Although the concept originated before the COVID-19 pandemic, its adoption surged during the pandemic, including temporary regulatory and reimbursement changes globally. These changes included:
- Allowing reimbursement of HaH care as a diagnosis-related group (DRG) in the US.
- Waiving certain previously mandatory requirements for remote patient monitoring.
- Encouraging telehealth and remote consulting.
- Allowing for remote data acquisition.
- Fast-tracking trials and vaccine entry into the market.
The UK, US, Spain, The Netherlands, Canada, and Australia have applied the HaH model, with early adoption in many other European countries. In the US, CMS has extended the acute HaH program, initiated in 2019, until December 2024 to further assess the benefits of HaH, showcasing a pioneering step toward telehealth adoption. A 2023 value assessment by the National Institute for Health and Care Excellence (NICE) in the UK supported the cost-effectiveness of HAH or virtual wards but acknowledged ongoing uncertainty and the need for further evidence. That review also highlighted assessment challenges like insufficient prior data, limited comparators, varied policies, and overall heterogeneity. However, NICE’s conditional approval supports generating more data and addressing unmet patient needs.
Challenges in the Adoption and Evaluation of DHT Suites
The grouping of DHTs, particularly those comprised of medical device (MD) and non-MD DHT components, presents safety and performance challenges due to networked-device interactions and novel system-level properties. Interoperability and applicability across various demographics also raise significant hurdles. Cybersecurity for individual and aggregated DHT systems, including internet of Medical Things devices (IoMT), requires careful regulatory attention, especially for systems like HaH, which form part of critical healthcare infrastructure. These systems could broaden risks beyond patients. Furthermore, the adaptive nature of many DHTs, whether through software updates or AI model training, adds complexity.
Under EU medical device regulations, combined systems of MDs and non-MD DHTs provided commercially are treated as MDs. The commercial provider must then conduct a specific risk assessment for all components, including clinical data for the combination. Based on this, manufacturers that utilize built-in smartphone/smartwatch components, such as ECG sensors, might need MD approval for all components due to their inclusion, even if they don’t make them. Guidance offers clarification, but this places the burden on app developers to assess and provide evidence for every possible sensor configuration, challenging given the sheer number of brands and sensors. As clinical settings become more complex, the question becomes: can regulatory standards realistically anticipate and test for every interaction scenario? Reimbursement is also a hurdle. Assessing cost-effectiveness and gathering evidence for DHT suites introduces new complexities, such as the absence of suitable comparators and the need to estimate initial investments for staff training and system development.
Conclusion
The transformation of healthcare through DHTs will largely come from novel DHT suites in interactive ‘fuzzy’ care networks involving HCPs, instead of single devices. The US Center for Devices and Radiological Health (CDRH) is actively developing strategies for a regulatory pathway that integrates this thinking. As the concept of DHT suites matures and the economic evaluations of these concepts continue, exploring whether new frameworks can be developed for the novel scenario of complex multi-device digital/human workflows presents a significant opportunity. The second article in this series will delve into what new frameworks could include.